Clinical Trial Management Software Development Company
Arkenea specializes in developing comprehensive clinical trial management systems (CTMS) that streamline research operations, ensure regulatory compliance, and accelerate time-to-market for pharmaceutical, biotechnology, and CRO organizations worldwide.

Awarded Best Healthcare Software Development Company in 2024 and 2025
Some of Our Clients






Leading Clinical Trial Management Software Development Company Since 2011
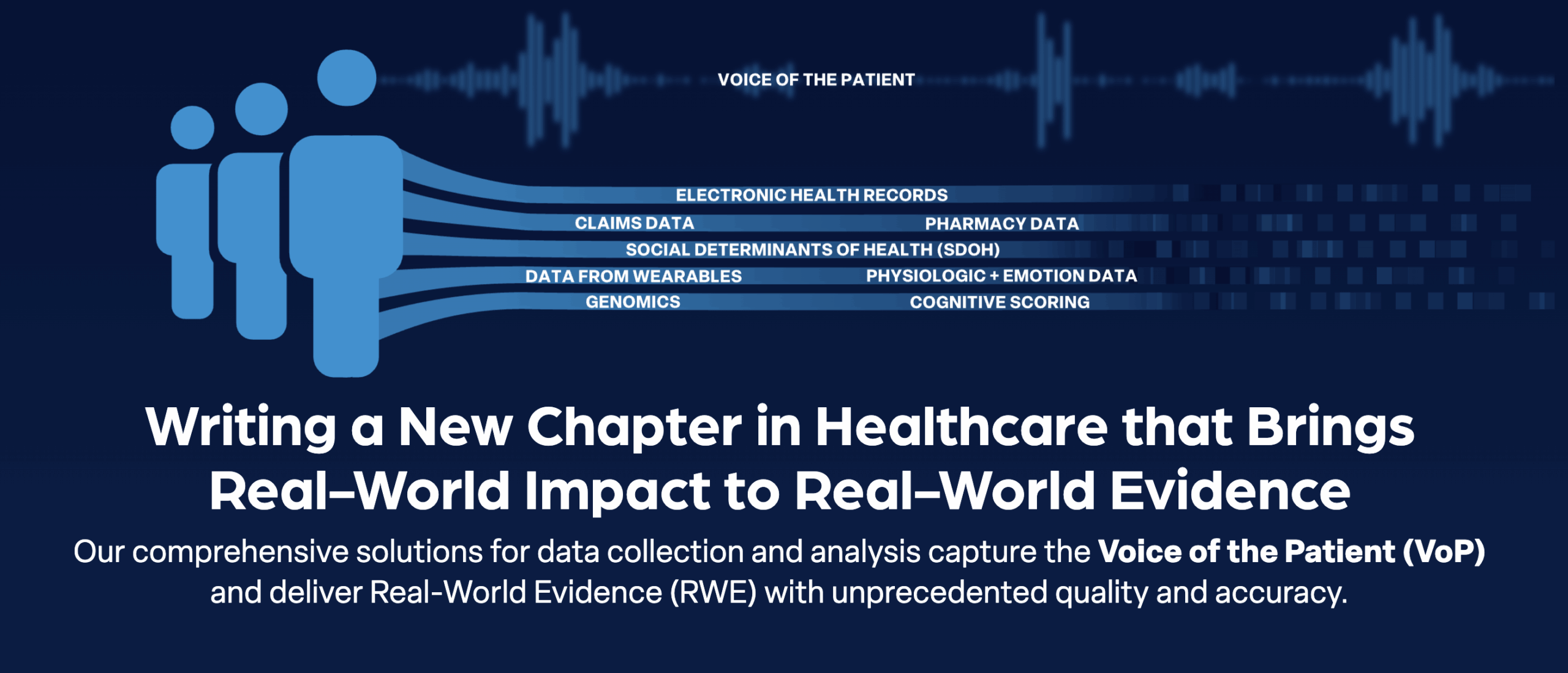
As the clinical trial landscape evolves toward decentralized and hybrid models, organizations require software development partners who understand not just technology, but the fundamental principles of clinical research. We help you develop innovative CTMS solutions that support traditional site based trials, fully decentralized studies, and hybrid approaches that combine the best of both models.
- 4.9 Clutch review rating with a 5.0 average referral rating.
- We help you develop a fully interoperable healthcare software leveraging FHIR and HL7 that can be integrated into by third-party healthcare systems and applications.
- Our API integration team helps seamlessly integrate your healthcare software with healthcare systems, EHRs and other third-party APIs, whether EHRs such as Oracle Cerner, EPIC or Athena Health or API engines such as 1upHealth and Redox Engine.
- We develop healthcare software compliant with HIPAA, HITRUST, HL7, IEC 62304, DICOM, ICD-10, PIPEDA, EPHI, PCI-DSS, IHE, LOINC, MDR, GMP and FDA 21 CFR Part 820 policies, where applicable.
- Developed CTMS solutions supporting over 500 clinical trials.
- Managed clinical trial data for 100,000+ study participants.
- Supported regulatory submissions to FDA, EMA, and 25+ international agencies.
Clinical Trial Industry Expertise
Pharmaceutical and Biotech Companies
Custom CTMS platforms for Phase I-IV trials, supporting complex study protocols, multi-site coordination, and regulatory compliance for drug development programs.
request a quoteContract Research Organizations
Scalable clinical trial management platforms that support multiple sponsors, standardized workflows, and efficient resource allocation across diverse therapeutic areas.
request a quoteAcademic Medical Centers
Integrated clinical research platforms that connect with existing hospital systems, support investigator initiated trials, and manage sponsored and institutional studies.
request a quoteMedical Device Companies
Specialized clinical trial software for device trials, including FDA IDE compliance, device tracking, and integration with medical device data collection systems.
request a quoteWhy Clients Trust Arkenea
Our clinical trial management software development expertise encompasses the full spectrum of clinical research technology needs, from protocol design and site management to data collection, monitoring, and regulatory reporting.
We understand the intricate requirements of Good Clinical Practice (GCP), the challenges of multi-site coordination, and the critical importance of data integrity in clinical research.
Every clinical trial management system we develop is architected with regulatory compliance as a foundational element, not an afterthought. Our deep understanding of FDA 21 CFR Part 11, EU Clinical Trials Regulation, ICH E6 (R2), and other international standards ensures your clinical trial software meets the highest regulatory requirements from day one.
Case Study
Case Study
MediMergent - Clinical Trial Management Software
Web-based CTMS Platform
A healthcare data company, MediMergent, partnered with us to build a custom clinical trial management platform that could handle the complexity of patient data. They needed a secure, HIPAA-compliant system that brought together multiple data types such as medical records, claims, prescriptions, physiological data, and patient reported outcomes into one centralized view. We designed a role based access system for different users like administrators, data managers, medical reviewers, and external contributors such as pharmacists and payors. The platform offered real time record tracking, detailed audit logs, and intelligent workflows to manage study protocols and patient adherence more efficiently.
One of the most powerful features of the system was its ability to process and interpret documents automatically. Using AWS Textract and Comprehend, the platform converted scanned medical records into structured data and used natural language processing to answer study-specific questions. If the system couldn’t confidently answer a threshold of those questions, the records were automatically flagged and sent to medical reviewers. This mix of automation and human oversight made the platform highly efficient and accurate. The entire system was built to scale, with built in flexibility for multi-tenant setups and support for evolving clinical trial needs. It’s now helping research teams focus less on data entry and more on delivering insights that drive better outcomes.

Comprehensive Clinical Trial Management Software Development Services
Custom Clinical Trial Management System (CTMS) Development
We develop custom CTMS solutions tailored to your specific clinical research needs and operational workflows. Our team builds platforms from the ground up based on your study requirements, therapeutic focus, and organizational processes. Whether you need a system for managing Phase I oncology trials or large scale cardiovascular studies, we create bespoke CTMS applications that integrate with your existing infrastructure and scale with your research portfolio growth.
Electronic Data Capture (EDC) Software Development
Our development team creates custom EDC applications designed around your unique study protocols and data collection requirements. We build tailored solutions that feature your specific form designs, validation rules, and workflow processes while ensuring regulatory compliance and data integrity. Each EDC system is developed to integrate seamlessly with your laboratory systems, imaging platforms, and reporting requirements, eliminating the limitations of off-the-shelf solutions.
Electronic Patient Reported Outcomes (ePRO) Application Development
We develop bespoke ePRO applications that align with your specific patient populations and study objectives. Our custom development approach allows us to create patient interfaces tailored to your therapeutic area, demographic requirements, and engagement strategies. These solutions are built to capture your unique outcome measures while providing the flexibility to adapt questionnaires and workflows based on your evolving research needs.
Decentralized Clinical Trial (DCT) Platform Development
Our team builds custom DCT platforms designed specifically for your hybrid or virtual trial requirements. We develop tailored solutions that incorporate your preferred telemedicine workflows, remote monitoring protocols, and patient engagement strategies. Each platform is architected based on your geographic reach, patient demographics, and regulatory requirements, ensuring optimal functionality for your specific decentralized trial objectives.
Clinical Research System Integration Development
We create custom integration solutions that connect your clinical trial systems with existing organizational infrastructure and third-party platforms. Our development team builds bespoke APIs and middleware based on your specific system requirements, whether connecting to laboratory networks, regulatory databases, or sponsor platforms. These custom integrations ensure seamless data flow while accommodating your unique operational processes and compliance requirements.
Regulatory Compliance Software Development
We develop clinical trial software solutions with built in regulatory compliance based on your specific jurisdictional and therapeutic area requirements. Our custom development approach ensures your applications meet FDA 21 CFR Part 11, ICH-GCP, and relevant CDISC standards while accommodating your organization’s unique validation and audit processes. Each solution is built with comprehensive documentation and testing protocols tailored to your regulatory submission needs.
Scalable Clinical Trial Technology Stack
Microservices Architecture
Enabling independent scaling of EDC, CTMS, and ePRO components.
API-First Design
Seamless integration with sponsor systems, central labs, and regulatory platforms.
Multi-Tenant SaaS Platforms
Supporting multiple studies, sites, and sponsors within a single instance
Real Time Data Processing
Immediate data validation, query generation, and safety signal detection.
Deep Clinical Research Expertise Across Therapeutic Areas
Oncology Clinical Trials
We have extensive experience developing CTMS solutions for oncology trials, including complex adaptive designs, biomarker-driven studies, and combination therapy protocols. Our systems support specialized oncology requirements such as RECIST criteria integration, adverse event reporting for cytotoxic agents, and patient-reported outcome measures specific to cancer treatment.
Rare Disease Research
Rare disease clinical trials require specialized approaches due to small patient populations, complex regulatory pathways, and unique endpoint considerations. We develop CTMS solutions that support patient registries, natural history studies, and adaptive trial designs optimized for rare disease research.
Cardiovascular Research
Our cardiovascular clinical trial expertise includes development of systems supporting large-scale outcomes trials, device studies, and complex endpoint adjudication processes. We understand the unique requirements of cardiovascular research including central ECG reading integration, clinical events committee workflows, and long-term safety follow-up protocols.
Pediatric Clinical Trials
Pediatric clinical research demands specialized considerations including age-appropriate consent processes, weight-based dosing calculations, and developmental milestone tracking. Our pediatric CTMS solutions incorporate these unique requirements while maintaining full regulatory compliance with pediatric research guidelines.
Proven Clinical Trial Software Development Methodology
1. Discovery and Requirement Analysis
Our architecture phase focuses on creating scalable, secure, and compliant system designs that can adapt to evolving clinical trial needs. We develop detailed technical specifications, database designs, integration architectures, and security frameworks that form the foundation for robust CTMS development.
2. UI/UX Design and Prototyping
Our designers craft intuitive and accessible interfaces that cater to both healthcare professionals and patients. We develop prototypes and wireframes, allowing stakeholders to provide early feedback before full scale development. The user experience is optimized across mobile, desktop, and wearable devices, ensuring accessibility and ease of use for all users.
3. Agile Development with Continuous Validation
We employ agile development methodologies adapted for regulated environments, with continuous validation, testing, and quality assurance throughout the development process. Our development teams include clinical research experts who ensure that technical solutions align with real-world clinical trial operations.
4. Compliance Implementation
We implement robust security measures at every stage of development. Our solutions adhere to HIPAA, FDA, and SOC 2 compliance requirements, incorporating data encryption, multi-factor authentication, and role-based access controls to safeguard sensitive patient information. We also integrate audit logging and real-time monitoring to proactively detect and prevent security breaches.
5. Integration with Healthcare Ecosystem
We enable HL7 and FHIR data exchange protocols, ensuring standardized connectivity between healthcare applications. Additionally, we integrate with wearable and IoT medical devices, allowing real time data collection for enhanced patient monitoring and diagnostics.
6. Quality Assurance
Our testing approach includes functional testing, performance testing, security testing, and user acceptance testing conducted in environments that mirror production clinical trial conditions. We perform comprehensive validation documentation to support regulatory compliance and system qualification.
7. Deployment
Our deployment process includes comprehensive user training, system documentation, and ongoing support to ensure successful adoption. We provide specialized training for different user roles including clinical research coordinators, data managers, and study monitors.
8. Ongoing Support and Enhancement
Post deployment support includes system monitoring, regular updates, regulatory compliance maintenance, and continuous enhancement based on user feedback and evolving clinical trial requirements.
FAQs for Developing a Custom Clinical Trial Management Software
What is clinical trial management software and why do organizations need it?
Clinical trial management software is a comprehensive digital platform that centralizes and streamlines all aspects of clinical research operations. Organizations conducting clinical trials face numerous challenges including complex regulatory requirements, multi site coordination, participant management, data collection, and safety monitoring. A well designed clinical trial management system addresses these challenges by providing integrated tools for protocol management, electronic data capture, participant tracking, regulatory reporting, and real time monitoring. The software becomes essential as trials grow in complexity, involve multiple sites, or require strict regulatory compliance. Modern clinical research demands sophisticated technology solutions that can handle the intricate workflows, data integrity requirements, and audit trail needs that manual processes simply cannot support effectively.
How long does it typically take to develop a custom clinical trial management system?
The development timeline for a custom clinical trial management system varies significantly based on the scope, complexity, and specific requirements of the project. A basic CTMS with core functionality typically requires 6 to 9 months for development, testing, and deployment. More comprehensive systems that include advanced features like electronic data capture, patient reported outcomes, risk based monitoring, and complex integrations usually take 12 to 18 months to complete. Large scale enterprise solutions with multiple modules, extensive customization, and integration with existing healthcare IT infrastructure can require 18 to 24 months or longer. The timeline also depends on factors such as regulatory validation requirements, the number of therapeutic areas supported, international compliance needs, and the level of customization required. Our development approach includes regular milestone reviews and iterative delivery to ensure the system meets evolving requirements throughout the development process.
What regulatory compliance standards must clinical trial software meet?
Clinical trial management software must comply with numerous regulatory standards depending on the geographic regions where trials will be conducted. In the United States, FDA 21 CFR Part 11 compliance is mandatory for electronic records and electronic signatures used in clinical trials. The software must also support Good Clinical Practice guidelines as outlined in ICH E6, which governs the conduct of clinical trials globally. European clinical trials must comply with the EU Clinical Trials Regulation and GDPR for data privacy protection. Additional standards include HIPAA for healthcare data protection, ISO 27001 for information security management, and various international guidelines such as ICH E2A for adverse event reporting. The software architecture must incorporate these compliance requirements from the ground up, including comprehensive audit trails, data integrity controls, user access management, and validation documentation. Our development process ensures that regulatory compliance is built into every aspect of the system rather than added as an afterthought.
Can clinical trial management software integrate with existing healthcare systems?
Yes, modern clinical trial management software is designed with integration capabilities as a core feature. The software can connect with electronic health record systems like Epic, Cerner, and Allscripts to streamline data flow between clinical care and research activities. Integration with laboratory information management systems enables automatic import of lab results, reducing manual data entry and improving accuracy. The software can also connect with imaging systems through DICOM integration, central laboratory platforms, electronic trial master file systems, and regulatory submission platforms. API based integration allows for real time data exchange while maintaining security and compliance standards. Our development approach prioritizes interoperability, ensuring that the CTMS becomes part of a connected healthcare ecosystem rather than an isolated system. This integration capability is particularly important for pragmatic clinical trials and real world evidence studies that rely on existing healthcare data sources.
What are the key features that should be included in a clinical trial management system?
A comprehensive clinical trial management system should include several essential features to support effective clinical research operations. Protocol management capabilities allow research teams to create, modify, and track study protocols while managing amendments and deviations. Participant management features include recruitment tracking, eligibility screening, informed consent management, visit scheduling, and retention monitoring. Electronic data capture functionality provides tools for creating case report forms, data entry, validation, and query management. Safety monitoring features include adverse event reporting, safety signal detection, and regulatory reporting capabilities. The system should also include randomization and trial supply management, financial tracking and budgeting tools, document management with version control, and comprehensive reporting and analytics dashboards. Advanced features might include risk based monitoring algorithms, patient reported outcome collection, mobile applications for site staff, and integration with wearable devices for digital biomarker collection. The specific feature set should be tailored to the organization's therapeutic areas, trial types, and operational requirements.
How much does it cost to develop custom clinical trial management software?
The cost of developing custom clinical trial management software varies widely based on the scope, complexity, and specific requirements of the project. CTMS development with core functionality typically ranges from $100,000 to $250,000. Factors that influence cost include the number of user roles supported, therapeutic area specialization, international regulatory requirements, integration complexity, mobile application development, and ongoing maintenance needs. The investment should be evaluated against the potential benefits including reduced trial timelines, improved data quality, regulatory compliance assurance, and operational efficiency gains. Our pricing approach is transparent and based on detailed requirements analysis to ensure clients understand the full scope and cost implications before development begins.
What is the difference between off the shelf and custom clinical trial management software?
Off the shelf clinical trial management software provides pre built functionality that can be implemented relatively quickly and at lower initial cost. These solutions offer standard features that work well for many organizations but may require workflow adaptation to fit the software's capabilities. Custom clinical trial management software is built specifically for an organization's unique requirements, workflows, and therapeutic focus areas. Custom solutions provide greater flexibility, can integrate seamlessly with existing systems, and can be modified as requirements evolve. The choice between off the shelf and custom solutions depends on factors such as budget, timeline, specific requirements, integration needs, and long term strategic goals. Organizations with unique workflows, specialized therapeutic areas, or complex integration requirements often benefit more from custom development. Custom solutions also provide competitive advantages through proprietary features and optimized workflows that off the shelf solutions cannot match.
How do you ensure data security and privacy in clinical trial software?
Data security and privacy in clinical trial software require comprehensive, multi layered approaches that address both technical and procedural aspects. Our development process implements end to end encryption for data transmission and storage, ensuring that sensitive clinical trial data remains protected throughout its lifecycle. Role based access controls limit system access to authorized personnel only, with detailed audit trails tracking all user activities. Multi factor authentication adds additional security layers, while regular security assessments and penetration testing identify potential vulnerabilities. The software architecture includes automated backup systems, disaster recovery capabilities, and data residency controls to meet international privacy requirements. GDPR compliance features include data subject rights management, consent tracking, and data minimization principles. HIPAA compliance is ensured through business associate agreements, administrative safeguards, and technical controls. Our security framework exceeds industry standards and undergoes regular third party audits to maintain the highest levels of data protection.
What support and maintenance services are provided after software deployment?
Comprehensive support and maintenance services are essential for the long term success of clinical trial management software. Our post deployment support includes 24/7 system monitoring to ensure optimal performance and immediate response to any technical issues. Regular software updates provide new features, security patches, and regulatory compliance updates as requirements evolve. User training programs ensure that research teams can effectively utilize all system capabilities, with ongoing training for new staff members. Technical support includes help desk services, troubleshooting assistance, and system optimization recommendations. Maintenance services cover database management, server maintenance, backup verification, and performance optimization. We also provide regulatory compliance monitoring to ensure the system continues to meet evolving regulatory requirements. Change management services help organizations adapt to new features and workflow improvements. Our support model is designed to minimize downtime, maximize system performance, and ensure that the clinical trial management software continues to deliver value throughout its operational lifecycle.
How can clinical trial management software improve trial efficiency and reduce costs?
Clinical trial management software significantly improves trial efficiency and reduces costs through automation, standardization, and real time monitoring capabilities. Automated workflows eliminate manual processes, reducing the time required for routine tasks like data entry, query generation, and report creation. Centralized data management improves data quality and reduces the need for extensive data cleaning activities. Real time monitoring capabilities enable early identification of issues before they impact trial timelines or data integrity. Electronic data capture eliminates paper based processes, reducing transcription errors and accelerating data availability. Integrated safety monitoring automates adverse event reporting and regulatory submissions, reducing compliance costs. Risk based monitoring algorithms optimize site monitoring activities, focusing resources on high risk areas while reducing overall monitoring costs. Patient engagement tools improve retention rates, reducing the costs associated with participant replacement. Comprehensive analytics provide insights that enable proactive decision making, preventing costly delays and protocol deviations. Organizations typically see 30 to 50 percent reductions in trial timelines and 20 to 40 percent reductions in operational costs after implementing comprehensive clinical trial management software.
